By Kyle Richardville
Understanding Ag, LLC
About the “Understanding” series
Agriculture isn’t rocket science. It’s much more complex than that. Farming and ranching involve the fields of biology, ecology, chemistry, botany, physics, geology, meteorology, politics, economics, psychology and mechanics, just to name a few. Companies make a fortune off many farmers and ranchers on such topics because it’s impossible to study everything and still have a life outside of work.
However, having a basic understanding of each of these topics has the potential to save a producer millions of dollars over his or her lifetime. This is why we are bringing you the “Understanding” series. The series’ purpose is to empower farmers and ranchers by helping them better understand the “why” behind regenerative practices so they can rely more on nature and less on expensive inputs.
What is pH and Why You Need to Know
A quick review of the pH basics
For anyone who might be having painful flashbacks of high school chemistry, there’s good news. pH is a really simple concept: It’s just the quantity of hydrogen atoms (H+) in a given place. That’s it. Very acidic conditions mean lots of H+, while very basic conditions mean very few H+. The pH scale goes from 0 (extremely acidic) to 14 (extremely alkaline), meaning 7 is neutral. A soil of 5.5 pH is considered acidic and has more H+ than one at a pH of 8, which is “basic” or “alkaline”.
In the soil, these H+ can be found in one of two places: floating in the water or clinging to soil solids like organic matter or clay, but less so with sand and silt. Soil solids have negative charges on their exterior, so the positive charge of the H+ sticks to soil exactly like the positive and negative poles of magnets stick together. The soil has plenty of other positively charged nutrients that do the same, including calcium (Ca2+), magnesium (Mg2+) and potassium (K+). Anything with a positive charge is called a “cation” in science-speak. (Just remember: Cat-ions have paws-itive charge) You may have heard of a soil’s “Cation Exchange Capacity” (CEC). This just refers to the total number of negative sites in the soil that cations can magnetically stick to. In many ways, cations compete with each other for these negatively-charged sites and can bump each other off, which is essentially how lime raises pH. More on that later.
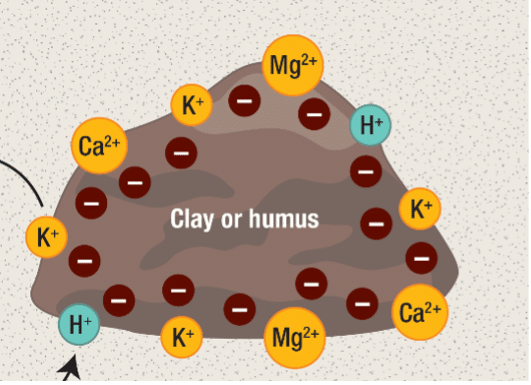
pH (a.k.a. the amount of H+ hanging around) is important to quantify because the compounds that make up living things begin to dissolve and break apart outside of a certain pH range. This is why the human body regulates blood pH to a miraculously tight range: 7.35-7.45. It’s also why our stomachs employ an acid with a pH of 2 to dissolve and break down the food we eat.
Without diving too deep into the controversy, traditional agronomic thinking is that nutrients become more or less available depending on pH. It’s taught that essential nutrients like iron (Fe), manganese (Mn), Boron (B) and aluminum (Al) are more available in acidic conditions, meaning they can build up to toxic levels in plants growing in low pH soils. Essential nutrients like molybdenum (Mo) and magnesium (Mg) are believed to be more available in basic conditions, meaning toxic levels can build up in plants growing in high pH soils. Innovative technology and experimentation are challenging this simplistic model of nutrient availability, meaning nutrient availability charts, like the one presented below, are likely overly simplistic.1
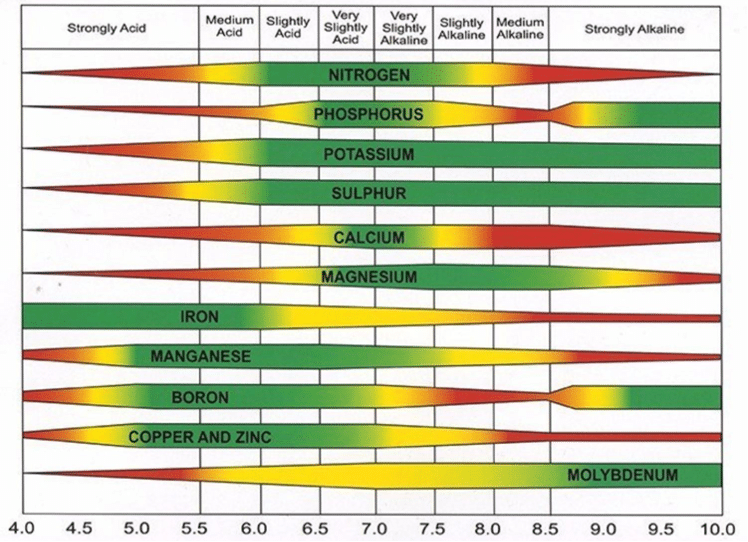
Natural Soil pH Levels
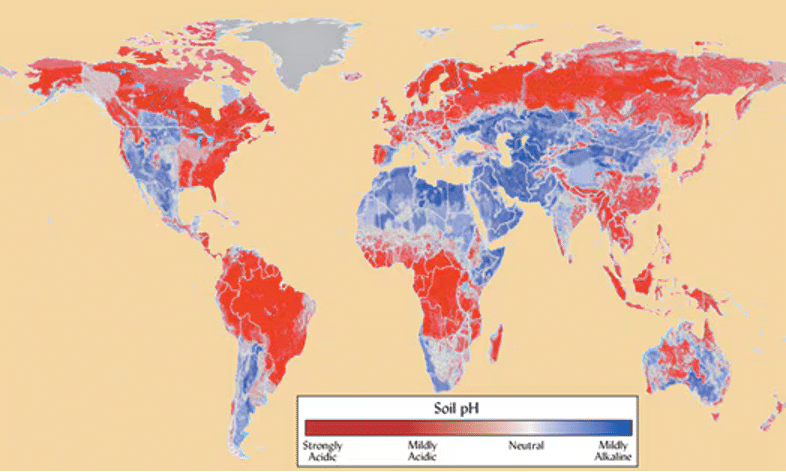
The truth is that soils around the world vary in their natural pH, largely related to annual rainfall. In higher rainfall areas, such as in the eastern US and land near the equator, soils slowly, but naturally, acidify. This occurs for a couple reasons. First, microbes excrete acids after they consume food. Those of you who have had cavities know this firsthand. Acids produced by bacteria in the mouth slowly decay tooth enamel. Sugary foods fuel microbial activity and result in more acid production, which is why candy does what it does to teeth. In the soil, moisture is often the limiting factor to microbial activity, so rain leads to a flurry of microbial feeding, which leads to acid production. In addition, microbes breathe out carbon dioxide (CO2) just like all living things, so more microbial activity means more is released.
This CO2 combines with water (H2O) to become a weak acid called carbonic acid (H2CO3), which can build up in the soil. All of these acids are not necessarily a bad thing. In fact, they are the reason why many nutrients are continually dissolved from rocks in the soil and made plant-available year after year. In addition, most agronomists agree that soils are more productive in the 6.3-6.8 range, meaning a slightly acidic environment. But the point here is soil moisture = more microbial activity = more acids.
Second, rain is naturally slightly acidic and adds H+ to the soil. Acid rain falling downwind of industrial processes acidifies soils much quicker, as was observed during the 19th and 20th centuries. Additional H+ from rain bumps positively charged nutrients like calcium (Ca2+), magnesium (Mg2+) and potassium (K+) off soil particles and leaves them at risk of leaching down through the soil and into the watershed. Losing these positively charged nutrients gives H+ more of a free reign and a chance to build up over time.
In contrast, soils in environments with less rainfall, such as in the western US, are at risk of slowly becoming alkaline. This is because less H+ is added to the soil from the rain. Low rainfall also means positively charged nutrients that compete with H+ do not leach out of the soil as quickly as they are dissolved from rocks in the soil by microbes. Therefore, the amount of calcium (Ca2+), magnesium (Mg2+), potassium (K+) and even sodium (Na+) increases over time and bumps out H+, resulting in a rise in pH.
Manmade Soil Acidification
The processes listed above naturally take a long time to play out and find an equilibrium. On the other hand, conventional management of cropland and pastureland has acidified many millions of acres unnaturally quickly in the past century or so, and this is the real issue at hand.
One cause of self-inflicted soil acidification is the excess use of nitrogen fertilizers like ammonium sulfate and urea because two H+ are released each time an ammonium (NH4+) ion is converted to nitrate (NO3–). This process happens in the soil naturally all the time, but the enormous dump of nitrogen fertilizer throws the process into hyperdrive, producing a large amount of H+. In addition, ammonium sulfate is broken down into sulfuric acid (H2SO4), a strong acid. Lastly, ever increasing crop yields over the decades has meant more calcium (Ca2+), magnesium (Mg2+) and potassium (K+) leaves the field at harvest, which has the same effect as them leaching in high rainfall areas because H+ is allowed to reign, and the balance of positively charged nutrients is thrown out of whack.
Various forms of nitrogen fertilizers affect soil pH differently, as can be seen in the chart below from North Dakota State University which shows how much lime is required to neutralize various fertilizers.2
| Nitrogen Source | Fertilizer Analysis (N-P-K) | Lime Required (lb CaCO3/lb N) |
| Anhydrous ammonia | 82-0-0 | 1.8 |
| Urea | 46-0-0 | 1.8 |
| Ammonium nitrate | 34-0-0 | 1.8 |
| Ammonium sulfate | 21-0-0-24 | 5.4* |
| Monoammonium phosphate | 11-52-0 | 5.4 |
| Diammonium phosphate | 18-46-0 | 3.6 |
| Urea-ammonium nitrate solutions | 28 to 32-0-0 | 1.8 |
Table 1: From Wortmann et al. (2015) as adapted from Havlin et al., 2005.
*The estimate for ammonium sulfate may be 50% too high (Chien et al., 2010)
Soil acidification also occurs in fields with high quantities of legumes like soybean and clover because enormous flushes of ammonium (NH4+) nitrogen are added to the soil when they decompose. This is yet another reason to prioritize diversity of species in cash crop rotations, cover crop mixes and pasture composition.
Another cause of soil pH problems is the slow loss of organic matter. Interestingly, organic matter does have a slightly acidifying effect as microbes decompose it and produce organic acids. However, as mentioned before, organic matter also provides crucial buffering effects of soil pH thanks to its numerous negatively charged sites that magnetically bind to H+ and other positively charged nutrients. Huge flushes of nitrogen or H+ don’t have as big of an effect on pH with high organic matter because they join a huge reservoir of positively charged nutrients already present. This is like putting $100 in a bank account with $100 versus putting $100 into a bank account with $100,000. The change in the first account is enormous but is only a small addition in the second. Such is the case with organic matter and its ability to moderate pH.
Soil organic matter also feeds and houses the trillions of microbes living in the soil that build soil structure. Proper soil structure contains macroaggregated soil particles and sufficient pore space for air and water exchange. These characteristics create a plethora of conditions in the soil, including areas that are dry, wet, anaerobic, aerobic, large, tiny and everything in between. A diversity of environments leads to an abundance and diversity of living creatures that thrive in each unique niche. Research in the area of microbial mediation of pH is in its infancy, but organic matter is known to improve problems with soil acidity3 and I suspect that a large portion of this occurs as a result of nutrient balancing and cycling by the trillions of living organisms working together in all of the different niches.
Manmade Soil Alkalinization
As mentioned previously, arid environments tend to produce alkaline soils over time because the quantity of cations like calcium (Ca2+), magnesium (Mg2+), potassium (K+) and even sodium (Na+) increases over time and bumps out H+ on negatively charged sites in the soil. Unfortunately, historical and current management by farmers and ranchers in these environments has sped up these nutrient buildups. The most common cause for these buildups is the excessive use of irrigation. Essentially, irrigation water from below contains a high concentration of these nutrients from the surrounding bedrock. This irrigation water lands on the surface and either gets taken up by plants or evaporates, leaving the nutrients behind in the form of white, salty material. This is the same reason why white specs of salt remain on our body after our sweat evaporates away. Similarly, another risk factor for the dangerous buildup of these nutrients and salts is poor drainage. High levels of these salts inhibit microbial and plant life over time.

Another management practice that has driven up soil pH and salt levels in arid regions is the replacement of deep-rooted perennial species with annual crop varieties. The reason is annual crops, along with fallow periods, decrease the amount of groundwater that is released through plant evapotranspiration. Over time, this causes the water table to rise, increasing the flow of nutrient-rich groundwater to low spots. This water will increasingly move toward the surface and results in high pH/salt buildup situations. As it stands, nearly 40 million acres of cropland and pasture in the western United States is negatively affected by buildup of these nutrients, and that number is growing annually.4
Next Time
With a basic (no pun intended) understanding of pH under our belts, the next installment in this series will discuss how lime works to works to raise soil pH in acidic soil conditions. In addition, we will cover how soil structure, nutrient balance and overall function are affected by this nearly universal amendment.
References
2 https://www.ndsu.edu/agriculture/extension/publications/what-soil-acidity
3 https://www.researchgate.net/publication/43518453_Role_of_organic_matter_in_alleviating_soil_acidity
